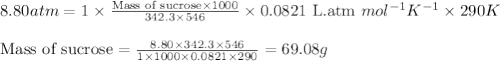Chemistry, 12.02.2020 03:24 cuppykittyy
What mass of sucrose (C12H22O11) should be combined with 546 g of water to make a solution with an osmotic pressure of 8.80 atm at 290 K ? (Assume the density of the solution to be equal to the density of the solvent.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
What mass of sucrose (C12H22O11) should be combined with 546 g of water to make a solution with an o...
Questions


History, 25.02.2021 20:20






Mathematics, 25.02.2021 20:20


Mathematics, 25.02.2021 20:20

Mathematics, 25.02.2021 20:20


Chemistry, 25.02.2021 20:20

Mathematics, 25.02.2021 20:20

Spanish, 25.02.2021 20:20


History, 25.02.2021 20:20


Arts, 25.02.2021 20:20

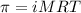
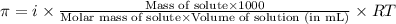
 = osmotic pressure of the solution = 8.80 atm
= osmotic pressure of the solution = 8.80 atm