
Chemistry, 12.02.2020 04:19 monsterenergy19
Check all the true statements concerning two identical containers, one with helium gas in it and one with xenon gas. Choose one or more:
a. When the temperature of either sample of gas increases, the root-mean-square speed increases.
b. If both gases are at the same temperature, they have the same average kinetic energy.
c. When the temperature of either sample of gas increases, the number of particles with average kinetic energy increases.
d. If both of the containers are at the same temperature, they will have the same root-mean-square speed.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

You know the right answer?
Check all the true statements concerning two identical containers, one with helium gas in it and one...
Questions


Mathematics, 22.08.2019 05:30

History, 22.08.2019 05:30

Biology, 22.08.2019 05:30

Biology, 22.08.2019 05:30

English, 22.08.2019 05:30

Mathematics, 22.08.2019 05:30


Mathematics, 22.08.2019 05:30


Geography, 22.08.2019 05:30



Mathematics, 22.08.2019 05:30


Biology, 22.08.2019 05:30



Business, 22.08.2019 05:30

Mathematics, 22.08.2019 05:30

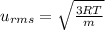
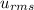 = root mean square speed
= root mean square speed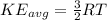 we see that the kinetic energy is directly proportional to its Kelvin temperature and hence b. is true
we see that the kinetic energy is directly proportional to its Kelvin temperature and hence b. is true

