
Chemistry, 12.02.2020 04:39 winterblanco
1. Use VSEPR theory to decide which one of the following ions and molecules is likely to be planar. (The central atom is always first in the formula.) A. BrF3 B. H3O C. PCl3 D. SO42- E. SF4

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1


Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

You know the right answer?
1. Use VSEPR theory to decide which one of the following ions and molecules is likely to be planar....
Questions

Mathematics, 17.01.2021 01:00



Mathematics, 17.01.2021 01:00


Computers and Technology, 17.01.2021 01:00


History, 17.01.2021 01:00



History, 17.01.2021 01:00

Mathematics, 17.01.2021 01:00

History, 17.01.2021 01:00

Social Studies, 17.01.2021 01:00

Mathematics, 17.01.2021 01:00

Mathematics, 17.01.2021 01:00

Mathematics, 17.01.2021 01:00

History, 17.01.2021 01:00


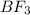
![\text{Number of electron pair}=\frac{1}{2}[V+N-C+A]](/tpl/images/0508/1323/9e987.png)
![\text{Number of electrons}=\frac{1}{2}\times [3+3]=3](/tpl/images/0508/1323/ce6da.png)
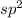 and the electronic geometry of the molecule will be trigonal planar.
and the electronic geometry of the molecule will be trigonal planar.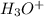
![\text{Number of electrons}=\frac{1}{2}\times [6+3-1]=4](/tpl/images/0508/1323/9514c.png)
 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.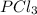
![\text{Number of electrons}=\frac{1}{2}\times [5+3]=4](/tpl/images/0508/1323/5f4d9.png)

![\text{Number of electrons}=\frac{1}{2}\times [6+2]=4](/tpl/images/0508/1323/2c46b.png)
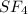
![\text{Number of electrons}=\frac{1}{2}\times [6+4]=5](/tpl/images/0508/1323/f1dca.png)
 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.

