Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1

Chemistry, 23.06.2019 05:30
How many moles are in 1.26*10^24 particles in significant figures
Answers: 2

Chemistry, 23.06.2019 09:50
T(s) in2os] (m) 0 185 2.39 546 1.90 725 1.70 the decomposition of n205 can be described by the equation 2.68 given these data for the reaction at 45°c in carbon tetrachloride solution, calculate the average rate of reaction for each successive time interval. ntr s to 185 s 185 s to 546 s 546 s to 725 s number number number reaction rate: m/s m/s m/s
Answers: 1

Chemistry, 23.06.2019 11:30
Bridget is in science class. her teacher gives her two unknown substances and asks her to determine their relative ph. she places a piece of red litmus paper into both substances. the litmus paper turns purple when she places it into substance i. the litmus paper turns blue when she places it into substance ii. a. substance i is a neutral substance and substance ii is an acid. b. substance i is a neutral substance and substance ii is a base. c. substance i is an acid and substance ii is a base. d. substance i is a base and substance ii is a neutral substance.
Answers: 1
You know the right answer?
A sample of octane burns releasing 2290 J of heat to the surroundings, and the gases produced expand...
Questions

Mathematics, 18.03.2021 03:10



Computers and Technology, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10


History, 18.03.2021 03:10


History, 18.03.2021 03:10

English, 18.03.2021 03:10


Health, 18.03.2021 03:10


Mathematics, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10


Mathematics, 18.03.2021 03:10



Mathematics, 18.03.2021 03:10

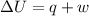
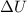 = internal energy
= internal energy