
Based on enthalpy of formation data species ∆H◦ f H2S(g) −20.63 kJ/mol O2(g) 0 kJ/mol H2O(ℓ) −285.83 kJ/mol SO2(g) −296.83 kJ/mol calculate ∆Hrxn for 2 H2O(ℓ) + 2 SO2(g) ←→ 2 H2S(g) + 3 O2(g) 1. 562 kJ · mol−1 2. −562 kJ · mol−1 3. −1124 kJ · mol−1 4. 1124 kJ · mol−1

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1
You know the right answer?
Based on enthalpy of formation data species ∆H◦ f H2S(g) −20.63 kJ/mol O2(g) 0 kJ/mol H2O(ℓ) −285.83...
Questions



Physics, 15.01.2021 17:20


History, 15.01.2021 17:20

English, 15.01.2021 17:20

Spanish, 15.01.2021 17:20

Social Studies, 15.01.2021 17:20

Chemistry, 15.01.2021 17:20


Mathematics, 15.01.2021 17:20

History, 15.01.2021 17:20

Mathematics, 15.01.2021 17:20

Health, 15.01.2021 17:20


English, 15.01.2021 17:20

Mathematics, 15.01.2021 17:20

Mathematics, 15.01.2021 17:20

Mathematics, 15.01.2021 17:20

Physics, 15.01.2021 17:20

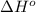
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f_{\text{(product)}}]-\sum [n\times \Delta H^o_f_{\text{(reactant)}}]](/tpl/images/0508/1357/eb0fa.png)
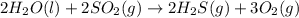
![\Delta H^o_{rxn}=[(2\times \Delta H^o_f_{(H_2S(g))})+(3\times \Delta H^o_f_{(O_2(g))})]-[(2\times \Delta H^o_f_{(H_2O(l))})+(2\times \Delta H^o_f_{(SO_2(g))})]](/tpl/images/0508/1357/e0170.png)
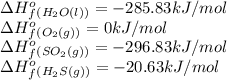
![\Delta H_{rxn}=[(2\times (-20.63))+(3\times (0))]-[(2\times (-285.83))+(2\times (-296.83))]\\\\\Delta H_{rxn}=1124kJ](/tpl/images/0508/1357/a0bfc.png)


