Consider the reaction
H2(g) + 1 2 O2(g) → H2O(ℓ) + 286 kJ
How much H2 would...

Chemistry, 12.02.2020 04:42 gabrielbergemancat
Consider the reaction
H2(g) + 1 2 O2(g) → H2O(ℓ) + 286 kJ
How much H2 would have to be burned to yield America’s daily energy share of 260,000 kcal? (1 cal = 4.184 J)
1.) 1900 mol
2.) 3800 mol
3.) 7600 mol
4.) 1 mol

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1
You know the right answer?
Questions

English, 21.02.2020 08:52

Mathematics, 21.02.2020 08:52


Mathematics, 21.02.2020 08:53

Mathematics, 21.02.2020 08:54


Mathematics, 21.02.2020 08:55

Mathematics, 21.02.2020 08:55

Physics, 21.02.2020 08:56




Mathematics, 21.02.2020 09:04

Mathematics, 21.02.2020 09:38




English, 21.02.2020 09:40


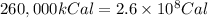 (Conversion factor: 1 kCal = 1000 Cal)
(Conversion factor: 1 kCal = 1000 Cal)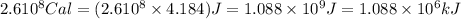
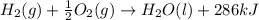
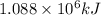 of energy will be released when
of energy will be released when 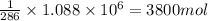 of hydrogen gas is consumed
of hydrogen gas is consumed

