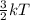A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon. Sort the conditions based on the gas described. Some may have more than one answer, but noe will have the same answer.
1) Nitrogen has the greater value
2) Xenon has the greater value
3) Both gases have the same value
A. Gas that exerts the greater partial pressure.
B. Gas for which the molecules or atoms have the greatest average velocity
C. Gas that would have a higher rate of effusion through a small hole opened in the flask.
D. Gas for which the molecules or atoms have the greatest average of kinetic energy.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

You know the right answer?
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon. Sort th...
Questions

Biology, 10.02.2021 07:20

World Languages, 10.02.2021 07:20



English, 10.02.2021 07:20

Mathematics, 10.02.2021 07:20

Chemistry, 10.02.2021 07:20



Mathematics, 10.02.2021 07:20

Mathematics, 10.02.2021 07:20




History, 10.02.2021 07:20




Mathematics, 10.02.2021 07:20

Biology, 10.02.2021 07:20