Chemistry, 12.02.2020 04:58 claudiseliss4910
Lithium oxide (Li2O) can be used to remove water from air. The balanced equation for the reaction of lithium oxide with water is: Li2O(s) + H2O(g) --> 2 LiOH(s) List the steps that you would have to follow to determine how many grams of water can be removed from the air by 250 g of Li2O, and identify the links between quantities. (Example: If you wanted to convert from μmol of a substance to grams, the steps would be μmol --> mol --> grams. The link between μmol and mol would be 1 μmol = 1E–6 mol, and the link between mol and grams would be the molar mass.)

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
Lithium oxide (Li2O) can be used to remove water from air. The balanced equation for the reaction of...
Questions


Mathematics, 15.11.2019 20:31

Mathematics, 15.11.2019 20:31

Mathematics, 15.11.2019 20:31

History, 15.11.2019 20:31

History, 15.11.2019 20:31

Mathematics, 15.11.2019 20:31





History, 15.11.2019 20:31

World Languages, 15.11.2019 20:31

Biology, 15.11.2019 20:31




Spanish, 15.11.2019 20:31

History, 15.11.2019 20:31

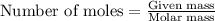 .....(1)
.....(1)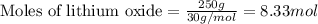
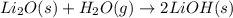
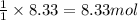 of water
of water