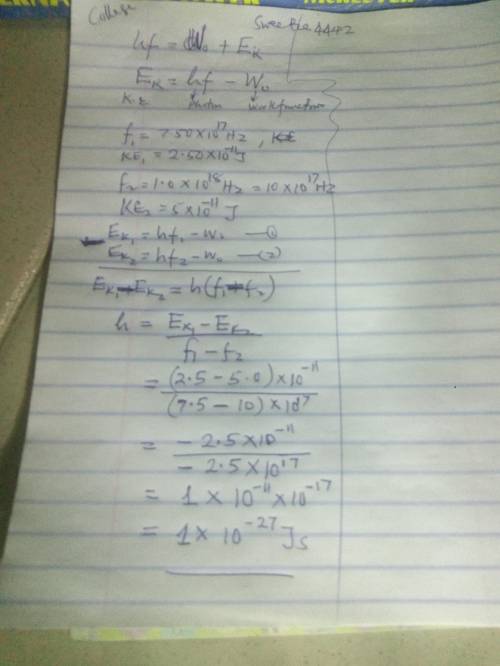The photoelectric effect can be used to measure the value of Planck’s constant. Suppose that a photoelectric effect experiment was carried out using light with ν = 7.50×1017 s-1 and ejected electrons were detected with a kinetic energy of 2.50×10-11 J. The experiment was then repeated using light with ν = 1.00×1018 s-1 and the same metal target, and electrons were ejected with a kinetic energy of 5.00×10-11 J. Use these data to find a value for Planck’s constant.
HINTS: these data are fictional and will give a result that is quite different from the real value of Planck’s constant. Be sure that you do not use the real value of Planck’s constant in any calculations here. It may help to start by thinking about how you would calculate the metal’s binding energy if you already knew Planck’s constant.
J s

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
The photoelectric effect can be used to measure the value of Planck’s constant. Suppose that a photo...
Questions

Mathematics, 24.07.2019 19:50



Mathematics, 24.07.2019 19:50

Mathematics, 24.07.2019 19:50

Health, 24.07.2019 19:50

Mathematics, 24.07.2019 20:00

Mathematics, 24.07.2019 20:00

History, 24.07.2019 20:00

Mathematics, 24.07.2019 20:00

Mathematics, 24.07.2019 20:00


Physics, 24.07.2019 20:00

Spanish, 24.07.2019 20:00



History, 24.07.2019 20:00


Mathematics, 24.07.2019 20:00