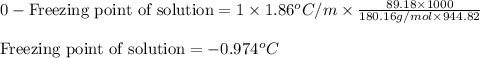Chemistry, 12.02.2020 18:31 gracebuffum
At 298 K, the osmotic pressure of a glucose solution (C6H12O6 (aq)) is 12.1 atm. Calculate the freezing point of the solution. The density of the solution is 1.034 g/mL.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 06:30
Generally, observed behavior that can be formulated into a statement, sometimes mathematical in nature, is called a(n): a. observation. b. measurement. c. theory. d. natural law. e. experiment.
Answers: 2
You know the right answer?
At 298 K, the osmotic pressure of a glucose solution (C6H12O6 (aq)) is 12.1 atm. Calculate the freez...
Questions


Mathematics, 12.02.2021 09:50

Mathematics, 12.02.2021 09:50

Business, 12.02.2021 09:50

Mathematics, 12.02.2021 09:50

Mathematics, 12.02.2021 09:50



History, 12.02.2021 09:50

English, 12.02.2021 09:50

Mathematics, 12.02.2021 09:50

English, 12.02.2021 09:50


Mathematics, 12.02.2021 09:50


Chemistry, 12.02.2021 09:50

Mathematics, 12.02.2021 09:50

Mathematics, 12.02.2021 09:50


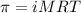
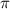 = osmotic pressure of the solution = 12.1 atm
= osmotic pressure of the solution = 12.1 atm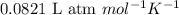
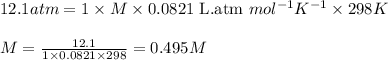
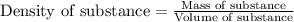
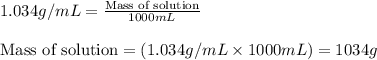
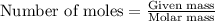
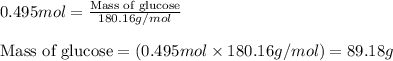
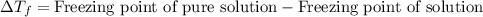
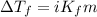
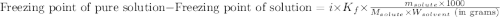
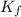 = molal freezing point elevation constant = 1.86°C/m
= molal freezing point elevation constant = 1.86°C/m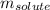 = Given mass of solute (glucose) = 89.18 g
= Given mass of solute (glucose) = 89.18 g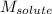 = Molar mass of solute (glucose) = 180.16 g/mol
= Molar mass of solute (glucose) = 180.16 g/mol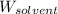 = Mass of solvent (water) = [1034 - 89.18] g = 944.82 g
= Mass of solvent (water) = [1034 - 89.18] g = 944.82 g