
Chemistry, 12.02.2020 18:46 59bobolucci
What is the mass, in grams, of each elemental sample? a. 2.3 * 10-3 molSb b. 0.0355 mol Ba c. 43.9 mol Xe d. 1.3 mol W

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1
You know the right answer?
What is the mass, in grams, of each elemental sample? a. 2.3 * 10-3 molSb b. 0.0355 mol Ba c. 43.9 m...
Questions

Chemistry, 05.05.2020 06:12

Chemistry, 05.05.2020 06:12

History, 05.05.2020 06:12

Mathematics, 05.05.2020 06:12




English, 05.05.2020 06:12


Mathematics, 05.05.2020 06:12


Mathematics, 05.05.2020 06:12




Mathematics, 05.05.2020 06:12

Mathematics, 05.05.2020 06:12

Mathematics, 05.05.2020 06:12

Mathematics, 05.05.2020 06:12

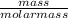
 mol
mol

