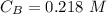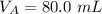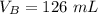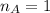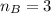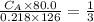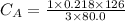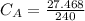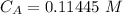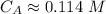Chemistry, 12.02.2020 20:06 liloleliahx2
The titration of 80.0 mL of an unknown concentration H3PO4 solution requires 126 mL of 0.218 M KOH solution. What is the concentration of the H3PO4 solution (in M)?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
The isotonic saline solution described in part a is connected to an unknown solution via a semipermeable membrane, the unknown solution level drops. based on this information, what can be said about these two solutions?
Answers: 1

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
You know the right answer?
The titration of 80.0 mL of an unknown concentration H3PO4 solution requires 126 mL of 0.218 M KOH s...
Questions

English, 30.03.2021 18:30




Chemistry, 30.03.2021 18:30

Mathematics, 30.03.2021 18:30



History, 30.03.2021 18:30

Social Studies, 30.03.2021 18:30



Chemistry, 30.03.2021 18:30


Mathematics, 30.03.2021 18:30

Mathematics, 30.03.2021 18:30

English, 30.03.2021 18:30


Mathematics, 30.03.2021 18:30

Physics, 30.03.2021 18:30

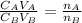
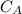 is the concentration of acid
is the concentration of acid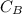 is the concentration of base
is the concentration of base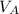 is the volume of acid
is the volume of acid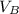 is the volume of base
is the volume of base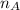 is the mole ratio of acid
is the mole ratio of acid 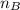 is the mole ratio of base
is the mole ratio of base