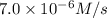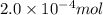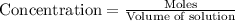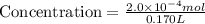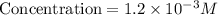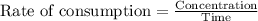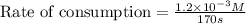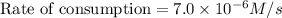Chemistry, 12.02.2020 20:10 catboy7196
If 2.0×10−4 moles of S2O2−8 in 170 mL of solution is consumed in 170 seconds , what is the rate of consumption of S2O2−8?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1
You know the right answer?
If 2.0×10−4 moles of S2O2−8 in 170 mL of solution is consumed in 170 seconds , what is the rate of c...
Questions


Spanish, 03.03.2021 02:40


Biology, 03.03.2021 02:40




Biology, 03.03.2021 02:40

History, 03.03.2021 02:40



Chemistry, 03.03.2021 02:40








Geography, 03.03.2021 02:40

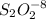 is,
is,