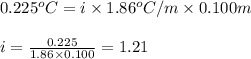Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3
You know the right answer?
The freezing-point depression of a 0.100 m MgSO4 solution is 0.225°C. Determine the experimental van...
Questions



Mathematics, 21.01.2021 17:40

Arts, 21.01.2021 17:40

Biology, 21.01.2021 17:40

History, 21.01.2021 17:40

Mathematics, 21.01.2021 17:40

English, 21.01.2021 17:40

Biology, 21.01.2021 17:40

Mathematics, 21.01.2021 17:40

Mathematics, 21.01.2021 17:40

History, 21.01.2021 17:40





Arts, 21.01.2021 17:40

Biology, 21.01.2021 17:40


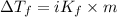
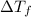 = depression in freezing point = 0.225°C
= depression in freezing point = 0.225°C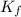 = Cryoscopic constant = 1.86°C/m
= Cryoscopic constant = 1.86°C/m