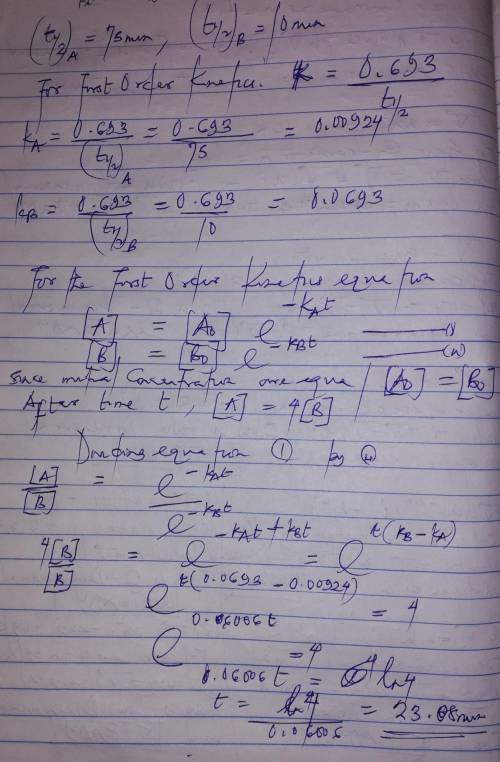Chemistry, 12.02.2020 22:50 sadieruegner393
A flask contains a mixture of compounds A and B. Both compounds decompose by first-order kinetics. The half-lives are 75.00 min for A and 10.00 min for B. If the concentrations of A and B are equal initially, how long will it take for the concentration of A to be four times that of B

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
A flask contains a mixture of compounds A and B. Both compounds decompose by first-order kinetics. T...
Questions








Mathematics, 02.06.2021 02:30







Chemistry, 02.06.2021 02:30


English, 02.06.2021 02:30