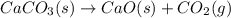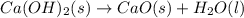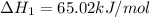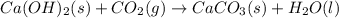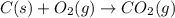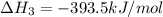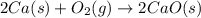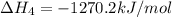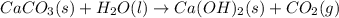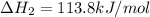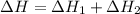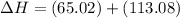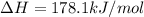Chemistry, 12.02.2020 23:10 makayladurham19
Determine the enthalpy change for the decomposition of calcium carbonate. CaCO3 (s) --> CaO (s) + CO2 (g) given the thermochemical equations below:
Ca(OH)2 (s) --> CaO (s) + H2O (l) enthalpy reaction = 65.2 kJ/mol
Ca(OH)2 (s) + CO2(g) --> CaCO3 (s) + H2O (l) enthalpy reaction = -113.8 kJ/mol
C(s) + O2 (g) --> CO2 (g) enthalpy of reation = -393.5 kJ/mol
2Ca(s) + O2(g) --> 2 CaO (s) enthalpy of reaction = -1270.2 kJ/mol
a. 1711.7 kJ/mol rxn
b. 441 kJ/mol rxn
c. 179 kJ/mol rxn
d. 48 kJ/mol rxn
e. 345.5 kJ. mol rxn

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2


Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1
You know the right answer?
Determine the enthalpy change for the decomposition of calcium carbonate. CaCO3 (s) --> CaO (s) +...
Questions

Mathematics, 28.08.2019 01:50

Mathematics, 28.08.2019 01:50



Mathematics, 28.08.2019 01:50

Mathematics, 28.08.2019 01:50


Biology, 28.08.2019 01:50





Biology, 28.08.2019 01:50

Mathematics, 28.08.2019 01:50

Social Studies, 28.08.2019 01:50



Mathematics, 28.08.2019 01:50

Social Studies, 28.08.2019 01:50

English, 28.08.2019 01:50