
Chemistry, 12.02.2020 23:28 purplepig12
If the freezing point of the solution had been incorrectly read 0.3 °C lower than the true freezing point, would the calculated molar mass of the solute have been too high or too low? Explain.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2


Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
If the freezing point of the solution had been incorrectly read 0.3 °C lower than the true freezing...
Questions

SAT, 04.08.2019 14:30



Mathematics, 04.08.2019 14:30


History, 04.08.2019 14:30


History, 04.08.2019 14:30

Physics, 04.08.2019 14:30

Physics, 04.08.2019 14:30

History, 04.08.2019 14:30

Mathematics, 04.08.2019 14:30



Chemistry, 04.08.2019 14:30

Mathematics, 04.08.2019 14:30

Mathematics, 04.08.2019 14:30


History, 04.08.2019 14:30

Chemistry, 04.08.2019 14:30

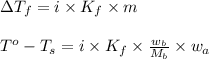
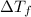 = change in freezing point
= change in freezing point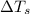 = freezing point of solution
= freezing point of solution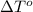 = freezing point of water
= freezing point of water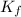 = freezing point constant
= freezing point constant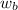 = mass of solute
= mass of solute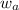 = mass of solvent
= mass of solvent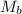 = molar mass of solute
= molar mass of solute

