Chemistry, 13.02.2020 00:34 halbrookc7082
A solution is prepared by mixing 0.10 L of 0.12 M sodium chloride with 0.23 L of a 0.18 M magnesium chloride solution. What volume of a silver nitrate solution (at 0.200 M) is required to precipitate all the Cl– ion in the solution as AgCl (s)?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

You know the right answer?
A solution is prepared by mixing 0.10 L of 0.12 M sodium chloride with 0.23 L of a 0.18 M magnesium...
Questions

Mathematics, 25.05.2021 16:50

Mathematics, 25.05.2021 16:50






Mathematics, 25.05.2021 16:50


Health, 25.05.2021 16:50

Biology, 25.05.2021 16:50





Mathematics, 25.05.2021 16:50

Mathematics, 25.05.2021 16:50


Mathematics, 25.05.2021 16:50

Biology, 25.05.2021 16:50

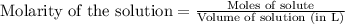
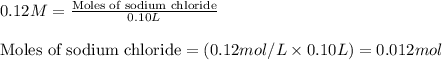
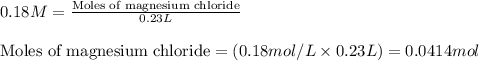
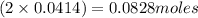
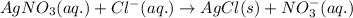
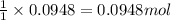 of silver nitrate
of silver nitrate