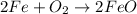A student reacts steel wool with oxygen by touching the fibers with a 9 volt battery. They begin
with 7.93 g Fe and measure the mass of the final product at 9.50 g.
The balanced equation for the reaction is:
2 Fe + 02 --> 2 FeO
A) The student claims that the percent yield of the reaction was 93.1 %. Support or reject their
claim including a calculation of percent yield as part of your evidence.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
You know the right answer?
A student reacts steel wool with oxygen by touching the fibers with a 9 volt battery. They begin
Questions


History, 27.06.2019 06:00

Mathematics, 27.06.2019 06:00

Mathematics, 27.06.2019 06:00


Mathematics, 27.06.2019 06:00

Business, 27.06.2019 06:00



History, 27.06.2019 06:00


English, 27.06.2019 06:00

Health, 27.06.2019 06:00

Mathematics, 27.06.2019 06:00

Mathematics, 27.06.2019 06:00




History, 27.06.2019 06:00