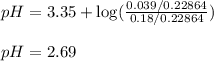Problem PageQuestion An analytical chemist is titrating of a solution of nitrous acid with a solution of . The of nitrous acid is . Calculate the pH of the acid solution after the chemist has added of the solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of solution added. Round your answer to decimal places.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 23.06.2019 03:00
Which of the following is a chemical property of water at 4 c
Answers: 2

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1

Chemistry, 23.06.2019 10:00
Lord kelvin described the concept of absolute zero temperature and the laws relating to the change of thermal energy during chemical reactions what type of chemist would he be considered today
Answers: 1
You know the right answer?
Problem PageQuestion An analytical chemist is titrating of a solution of nitrous acid with a solutio...
Questions


Mathematics, 13.10.2020 06:01

Biology, 13.10.2020 06:01


Biology, 13.10.2020 06:01


Social Studies, 13.10.2020 06:01

Mathematics, 13.10.2020 06:01

English, 13.10.2020 06:01

Physics, 13.10.2020 06:01



Mathematics, 13.10.2020 06:01


History, 13.10.2020 06:01




History, 13.10.2020 06:01

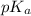 of nitrous acid is 3.35. Calculate the pH of the acid solution after the chemist has added 46.44 mL of the KOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of solution added. Round your answer to 2 decimal places.
of nitrous acid is 3.35. Calculate the pH of the acid solution after the chemist has added 46.44 mL of the KOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of solution added. Round your answer to 2 decimal places.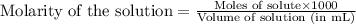
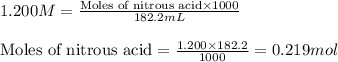


![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0509/3412/e4eea.png)
![pH=pK_a+\log(\frac{[KNO_2]}{[HNO_2]})](/tpl/images/0509/3412/fa3ae.png)
![[KNO_2]=\frac{0.039}{0.22864}](/tpl/images/0509/3412/7262b.png)
![[HNO_2]=\frac{0.18}{0.22864}](/tpl/images/0509/3412/eee2b.png)