Chemistry, 13.02.2020 02:23 bonetanne7171
The density at 20 ∘C of a 0.828 M solution of acetic acid in water is 1.0052 g/mL. The molar mass of acetic acid, CH3CO2H, is 60.05 g/mol. What is the molality of the solution?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1
You know the right answer?
The density at 20 ∘C of a 0.828 M solution of acetic acid in water is 1.0052 g/mL. The molar mass of...
Questions


Mathematics, 07.12.2021 20:20

Social Studies, 07.12.2021 20:20

English, 07.12.2021 20:20




History, 07.12.2021 20:20



Social Studies, 07.12.2021 20:20



Mathematics, 07.12.2021 20:20

Mathematics, 07.12.2021 20:20


Spanish, 07.12.2021 20:20


Computers and Technology, 07.12.2021 20:20

![d=M[\frac{1}{m}+\frac{M_b}{1000}]](/tpl/images/0509/4030/a25dd.png)
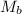 = molar mass of solute (acetic acid) = 60.05 g/mole
= molar mass of solute (acetic acid) = 60.05 g/mole![1.0052g/ml=0.828mol/L\times [\frac{1}{m}+\frac{60.05g/mole}{1000}]](/tpl/images/0509/4030/d7754.png)