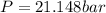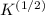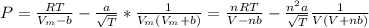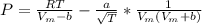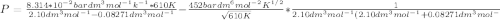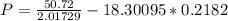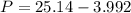Chemistry, 13.02.2020 04:53 Gearyjames8
Calculate the pressure exerted by benzene for a molar volume of 2.10 L at 610. K using the Redlich-Kwong equation of state: P==RTVm−b−aT√1Vm(Vm+b)nRTV−nb−n2aT√ 1V(V+nb) The Redlich-Kwong parameters a and b for benzene are 452.0 bar⋅dm6⋅mol−2⋅K1/2 and 0.08271 dm3⋅mol−1, respectively. Express your answer with the appropriate units.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2


Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
Calculate the pressure exerted by benzene for a molar volume of 2.10 L at 610. K using the Redlich-K...
Questions


Mathematics, 08.01.2021 22:10

Computers and Technology, 08.01.2021 22:10


Social Studies, 08.01.2021 22:10

Chemistry, 08.01.2021 22:10


Mathematics, 08.01.2021 22:10

Social Studies, 08.01.2021 22:10





Mathematics, 08.01.2021 22:10

Mathematics, 08.01.2021 22:10

Chemistry, 08.01.2021 22:10

Mathematics, 08.01.2021 22:10

English, 08.01.2021 22:10