
Chemistry, 13.02.2020 05:55 jasonoliva13
Which of the following sets are isoelectronic (i. e., have the same number of electrons)? i. Br-, Kr, Sr2+ ii. C, N-, O2- iii. Mg2+, Ca2+, Sr2+ iv. O2-, O, O2+ v. Ag+, Cd2+, Pd

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
Which of the following sets are isoelectronic (i. e., have the same number of electrons)? i. Br-, Kr...
Questions

Biology, 25.01.2021 01:00

Mathematics, 25.01.2021 01:00




Mathematics, 25.01.2021 01:00




Mathematics, 25.01.2021 01:00


English, 25.01.2021 01:00

Health, 25.01.2021 01:00

English, 25.01.2021 01:00


Mathematics, 25.01.2021 01:00

Mathematics, 25.01.2021 01:00

Arts, 25.01.2021 01:00

Mathematics, 25.01.2021 01:00

Mathematics, 25.01.2021 01:00

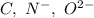
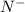 has 6 electrons.
has 6 electrons.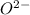 has 6 electrons.
has 6 electrons.

