Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
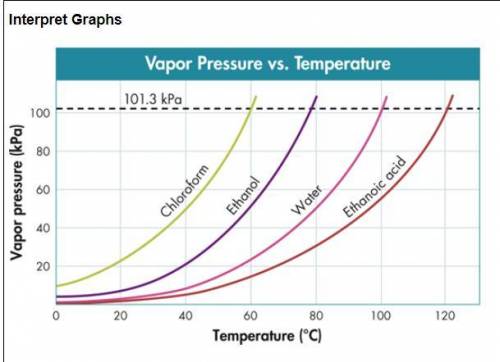
Use the figure below to determine to boiling point of -Chloroform at 80 kPa -Ethanol at 20 kPa -Etha...
Questions





Business, 21.01.2022 14:00


Mathematics, 21.01.2022 14:00





Medicine, 21.01.2022 14:00

Arts, 21.01.2022 14:00

History, 21.01.2022 14:00


Health, 21.01.2022 14:00

Mathematics, 21.01.2022 14:00


Mathematics, 21.01.2022 14:00

Mathematics, 21.01.2022 14:00