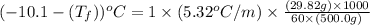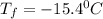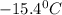A certain substance X has a normal freezing point of -10.1 degree C and a molal freezing point depression constant K_f = 5.32 degree C middot kg middot mol^-1. Calculate the freezing point of a solution made of 29.82 g of urea ((NH_2)_CO) dissolved in 500. g of X. Be sure your answer has the correct number of significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Measuring which physical property is most likely to produce the most precise results when trying to identify a substance
Answers: 1

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
A certain substance X has a normal freezing point of -10.1 degree C and a molal freezing point depre...
Questions




English, 16.06.2021 18:50


History, 16.06.2021 18:50

Mathematics, 16.06.2021 18:50

Business, 16.06.2021 18:50

English, 16.06.2021 18:50




Mathematics, 16.06.2021 18:50

Mathematics, 16.06.2021 18:50

Mathematics, 16.06.2021 18:50

Mathematics, 16.06.2021 18:50


English, 16.06.2021 18:50


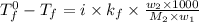
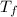 = boiling point of solution = ?
= boiling point of solution = ?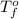 = boiling point of solvent (X) =
= boiling point of solvent (X) = 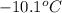
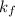 = freezing point constant =
= freezing point constant = 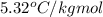
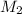 = molar mass of solute (urea) = 60 g/mol
= molar mass of solute (urea) = 60 g/mol