1. ClO-(aq) + H2O(l) => HClO(aq) + OH-(aq) SLOW

Chemistry, 13.02.2020 18:36 samantha9430
The proposed mechanism for a reaction is
1. ClO-(aq) + H2O(l) => HClO(aq) + OH-(aq) SLOW
2. I-(aq) + HClO(aq) => HIO(aq) + Cl-(aq) FAST
3. OH-(aq) + HIO(aq) => H2O(l) + IO-(aq) FAST
Which of the following would be a rate law for the reaction?
1. rate = k[ClO-][H2O][I-][OH-]
2. rate = k[ClO-][H2O]
3. rate = k[OH-][HIO]
4. rate = k[I-][HClO]
5. rate = k[ClO-][H2O][I-]

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
You know the right answer?
The proposed mechanism for a reaction is
1. ClO-(aq) + H2O(l) => HClO(aq) + OH-(aq) SLOW
1. ClO-(aq) + H2O(l) => HClO(aq) + OH-(aq) SLOW
Questions

Mathematics, 04.02.2021 20:10

French, 04.02.2021 20:10


Spanish, 04.02.2021 20:10

Mathematics, 04.02.2021 20:10

Mathematics, 04.02.2021 20:10

Computers and Technology, 04.02.2021 20:10

Mathematics, 04.02.2021 20:10



Mathematics, 04.02.2021 20:10

Biology, 04.02.2021 20:10

Mathematics, 04.02.2021 20:10

Mathematics, 04.02.2021 20:10

Mathematics, 04.02.2021 20:10


Mathematics, 04.02.2021 20:10

Mathematics, 04.02.2021 20:10

Advanced Placement (AP), 04.02.2021 20:10

History, 04.02.2021 20:10

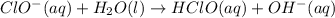 is a slow step reaction.
is a slow step reaction.![k[ClO^{-}][H_{2}O]](/tpl/images/0510/0437/87324.png)


