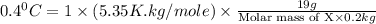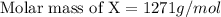Chemistry, 13.02.2020 18:46 kingdrex4772
When 19g of a certain molecular compound X are dissolved in of benzonitrile , the freezing point of the solution is measured to be . Calculate the molar mass of X.

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
When 19g of a certain molecular compound X are dissolved in of benzonitrile , the freezing point of...
Questions

Mathematics, 04.03.2021 17:30




Mathematics, 04.03.2021 17:30

Mathematics, 04.03.2021 17:30


English, 04.03.2021 17:30

Mathematics, 04.03.2021 17:30

Advanced Placement (AP), 04.03.2021 17:30

Biology, 04.03.2021 17:30

Chemistry, 04.03.2021 17:30

Mathematics, 04.03.2021 17:30

Mathematics, 04.03.2021 17:30


Mathematics, 04.03.2021 17:30

Mathematics, 04.03.2021 17:30

Computers and Technology, 04.03.2021 17:30

Mathematics, 04.03.2021 17:30

Computers and Technology, 04.03.2021 17:30

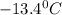 . Calculate the molar mass of X. Freezing point of pure benzonitrile =
. Calculate the molar mass of X. Freezing point of pure benzonitrile = 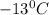
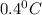
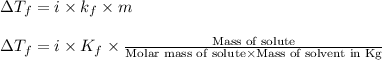
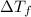 = change in freezing point =
= change in freezing point = 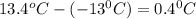
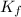 = freezing point constant = 5.35 K.kg/mole
= freezing point constant = 5.35 K.kg/mole