
Chemistry, 13.02.2020 18:57 jamilamiller200
When an antacid tablet dissolves in water, the fizz is due to a reaction between sodium hydrogen carbonate (sodium bicarbonate, NaHCO3) and citric acid (H3C6H5O7). 3 NaHCO3(aq) H3C6H5O7(aq) 3 CO2(g) 3 H2O(l) Na3C6H5O7(aq) How many moles of Na3C6H5O7 can be produced if one tablet containing 0.0370 mol of NaHCO3 is dissolved

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
When an antacid tablet dissolves in water, the fizz is due to a reaction between sodium hydrogen car...
Questions

Mathematics, 04.05.2020 22:57

Mathematics, 04.05.2020 22:57

History, 04.05.2020 22:57


Geography, 04.05.2020 22:57

Biology, 04.05.2020 22:58


Mathematics, 04.05.2020 22:58

Mathematics, 04.05.2020 22:58

Mathematics, 04.05.2020 22:58

Biology, 04.05.2020 22:58


Mathematics, 04.05.2020 22:58

Mathematics, 04.05.2020 22:58

History, 04.05.2020 22:58

History, 04.05.2020 22:58


English, 04.05.2020 22:58


English, 04.05.2020 22:58

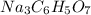 produced can be, 0.0123 mole
produced can be, 0.0123 mole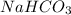 = 0.0370 mol
= 0.0370 mol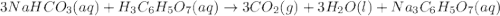
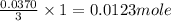 of
of 

