
Chemistry, 13.02.2020 19:57 CrusaderLord
For the reaction Fe3+(aq) + SCN-(aq) Fe(SCN)2+(aq). a. Addition of SCN-(aq) will shift the equilibrium in favor of products. b. Addition of SCN-(aq) will shift the equilibrium in favor of reactants. c. Addition of Cl-(aq) will shift the equilibrium in favor of reactants.

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1

Chemistry, 24.06.2019 01:00
Consider the chemical equilibrium of the reaction. agcl(s) ag+(aq) + cl–(aq) what will happen to the chemical equilibrium if agno3 is added? a there is no shift in the chemical equilibrium of the system. b the chemical equilibrium of the system shifts to the right. c the chemical equilibrium of the system shifts to the left. d the chemical equilibrium of the system is destroyed.
Answers: 1

You know the right answer?
For the reaction Fe3+(aq) + SCN-(aq) Fe(SCN)2+(aq). a. Addition of SCN-(aq) will shift the equilibri...
Questions

Mathematics, 06.10.2019 18:30

Mathematics, 06.10.2019 18:30

Chemistry, 06.10.2019 18:30




Mathematics, 06.10.2019 18:30

Mathematics, 06.10.2019 18:30

History, 06.10.2019 18:30


Advanced Placement (AP), 06.10.2019 18:30


Mathematics, 06.10.2019 18:30


Mathematics, 06.10.2019 18:30

Biology, 06.10.2019 18:30

Mathematics, 06.10.2019 18:30

English, 06.10.2019 18:30


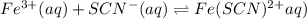
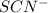 is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of
is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of 

