Chemistry, 13.02.2020 20:15 kkelley9223
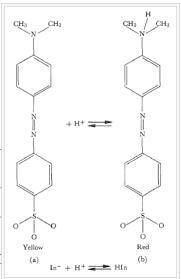
Methyl orange (HMO) is an acid-base indicator. Its two forms in solution are HMO (red) and MO- (yellow). When HMO is added to distilled water, the solution is yellow. How would you turn the solution red? a. add acid b. add base c. add distilled water

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
Methyl orange (HMO) is an acid-base indicator. Its two forms in solution are HMO (red) and MO- (yell...
Questions

Mathematics, 02.06.2020 06:58

Mathematics, 02.06.2020 06:58

Mathematics, 02.06.2020 06:58

Mathematics, 02.06.2020 06:58

Mathematics, 02.06.2020 06:58

Mathematics, 02.06.2020 06:58


Health, 02.06.2020 06:58

Mathematics, 02.06.2020 06:58


Mathematics, 02.06.2020 06:58

Mathematics, 02.06.2020 06:58

Mathematics, 02.06.2020 06:58

Mathematics, 02.06.2020 06:58

Mathematics, 02.06.2020 06:58



Mathematics, 02.06.2020 06:58

Mathematics, 02.06.2020 06:58

Biology, 02.06.2020 06:58