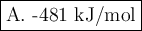Chemistry, 13.02.2020 20:53 camcollins00
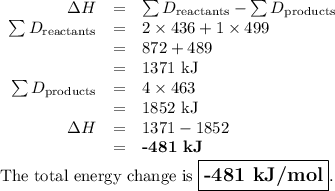
What is the total energy change for the following reaction: 2H2 + O2 -> 2H2O?
Given:
H-H bond: 436 kJ/mol
O-O double bond: 499 kJ/mol
H-O bond: 463 kJ/mol
A. -481 kJ/mol
B. + 445 kJ/mol
C. +63 kJ/mol
D. -730.5 kJ/mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1

Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2
You know the right answer?
What is the total energy change for the following reaction: 2H2 + O2 -> 2H2O?
Given:...
Given:...
Questions

Mathematics, 17.08.2020 01:01






Mathematics, 17.08.2020 01:01







Geography, 17.08.2020 01:01






 = ( A ) ; - 481 kJ/mol
= ( A ) ; - 481 kJ/mol