
Chemistry, 13.02.2020 20:57 mapoohdoll
Initially, a closed flask contains a mixture of N2 at a partial pressure of 3 atm and H2 at a partial pressure of 5 atm. The mixture reaches equilibrium and the partial pressure of NH3 is 2 atm. What is the value of the equilibrium constant?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1
You know the right answer?
Initially, a closed flask contains a mixture of N2 at a partial pressure of 3 atm and H2 at a partia...
Questions



English, 19.09.2019 19:30

History, 19.09.2019 19:30


History, 19.09.2019 19:30

Health, 19.09.2019 19:30


History, 19.09.2019 19:30

Chemistry, 19.09.2019 19:30



Social Studies, 19.09.2019 19:30

Mathematics, 19.09.2019 19:30



Chemistry, 19.09.2019 19:30


English, 19.09.2019 19:30

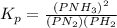 Hence we have
Hence we have


