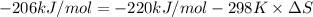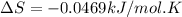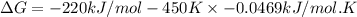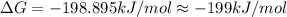For the gaseous reaction of carbon monoxide and chlorine to form phosgene (COCL2)
perform the following calculations
A) calculate delta S at 298k ( delta H= -220.kj/mol and delta G= -206kj/mol
B) assuming that delta S and delta H change little with temperature, calculate at 450k

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1
You know the right answer?
For the gaseous reaction of carbon monoxide and chlorine to form phosgene (COCL2)
perfor...
perfor...
Questions


Mathematics, 21.06.2020 23:57








Geography, 21.06.2020 23:57

Mathematics, 21.06.2020 23:57


English, 21.06.2020 23:57


Mathematics, 21.06.2020 23:57

Mathematics, 21.06.2020 23:57




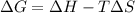
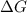 = Gibbs free energy = -206 kJ/mol
= Gibbs free energy = -206 kJ/mol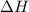 = enthalpy change = -220 kJ/mol
= enthalpy change = -220 kJ/mol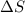 = entropy change = ?
= entropy change = ?