Chemistry, 13.02.2020 22:22 haileyrae187
One mole of an ideal gas is contained in a cylinder with a movable piston. The temperature is constant at 778C. Weights are removed suddenly from the piston to give the following sequence of three pressures: a. P1 5 5.00 atm (initial state) b. P2 5 2.24 atm c. P3 5 1.00 atm (final state)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
You know the right answer?
One mole of an ideal gas is contained in a cylinder with a movable piston. The temperature is consta...
Questions

English, 07.12.2021 09:20

Mathematics, 07.12.2021 09:20

History, 07.12.2021 09:20




English, 07.12.2021 09:20


Mathematics, 07.12.2021 09:20



Mathematics, 07.12.2021 09:20

Engineering, 07.12.2021 09:20


Physics, 07.12.2021 09:20

Mathematics, 07.12.2021 09:20

History, 07.12.2021 09:20


History, 07.12.2021 09:20

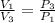
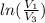 =
= 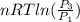
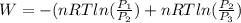 or
or 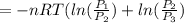
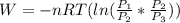 =
= 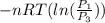 therefore the equation is the same for calculating directly from the initial pressure P₁, to the final pressure P₃
therefore the equation is the same for calculating directly from the initial pressure P₁, to the final pressure P₃