
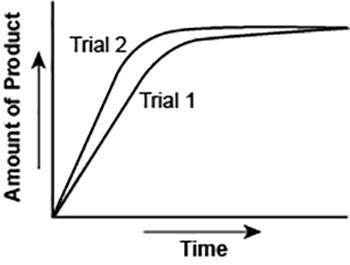
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different concentration of reactant was used during each trial, whereas the other factors were kept constant.
Which of the following statements explains which trial has a *lower* concentration of the reactant? (5 points)
Trial 1, because the average rate of the reaction is lower.
Trial 1, because this reaction lasted for a longer duration than Trial 2.
Trial 2, because this reaction was initially fast and later slowed down.
Trial 2, because the volume of product formed per unit time was higher.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2


Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different...
Questions

English, 28.08.2020 03:01







Mathematics, 28.08.2020 03:01

Mathematics, 28.08.2020 03:01


English, 28.08.2020 03:01

Biology, 28.08.2020 03:01




History, 28.08.2020 03:01

Spanish, 28.08.2020 03:01

Mathematics, 28.08.2020 03:01

Mathematics, 28.08.2020 03:01

Mathematics, 28.08.2020 03:01



