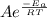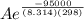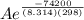The standard free energy of activation of one reaction A is 95.00 kJ mol–1 (22.71 kcal mol–1). The standard free energy of activation of another reaction B is 74.20 kJ mol–1 (17.73 kcal mol–1). Assume a temperature of 298 K and 1 M concentration.
By what factor is one reaction faster than the other?
Which reaction is faster?
(a) Reaction A is faster.
(b) Reaction B is faster.
(c) Cannot be determined.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1

You know the right answer?
The standard free energy of activation of one reaction A is 95.00 kJ mol–1 (22.71 kcal mol–1). The s...
Questions


Business, 26.07.2019 20:30






Physics, 26.07.2019 20:30

English, 26.07.2019 20:30




Mathematics, 26.07.2019 20:30


Mathematics, 26.07.2019 20:30

Computers and Technology, 26.07.2019 20:30

Mathematics, 26.07.2019 20:30

English, 26.07.2019 20:30

Mathematics, 26.07.2019 20:30

Health, 26.07.2019 20:30