
Chemistry, 14.02.2020 01:41 katwright1124
Consider the following system at equilibrium where H° = -111 kJ, and Kc = 0.159 , at 723 K: N2 (g) + 3 H2 (g) 2 NH3 (g)
If the TEMPERATURE on the equilibrium system is suddenly decreased :
The value of Kc A. Increases B. Decreases C. Remains the same
The value of QcA. Is greater than Kc B. Is equal to Kc C. Is less than Kc
The reaction must: A. Run in the forward direction to restablish equilibrium. B. Run in the reverse direction to restablish equilibrium. C. Remain the same. Already at equilibrium.
The concentration of H2 will: A. Increase. B. Decrease. C. Remain the same.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1


Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
Consider the following system at equilibrium where H° = -111 kJ, and Kc = 0.159 , at 723 K: N2 (g) +...
Questions

Biology, 24.07.2019 07:30

Biology, 24.07.2019 07:30


Biology, 24.07.2019 07:30


Biology, 24.07.2019 07:30


Biology, 24.07.2019 07:30



Biology, 24.07.2019 07:30



Chemistry, 24.07.2019 07:30






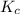 will increase.
will increase.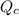 will decrease.
will decrease.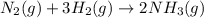
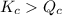 ( Move in froward direction )
( Move in froward direction )


