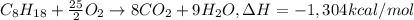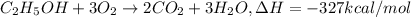Chemistry, 14.02.2020 02:02 blackjack73
Ethanol is added to gasoline to produce E-15 and E-85. It promotes more complete combustion of the gasoline and is an octane booster. Compare the heats of combustion of 2,2,4-trimethylpentane (1304 kcal/mol) and ethanol (327 kcal/mol). Which has the higher heat of combustion in kcal/mol?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1

You know the right answer?
Ethanol is added to gasoline to produce E-15 and E-85. It promotes more complete combustion of the g...
Questions

Computers and Technology, 08.04.2020 04:29


English, 08.04.2020 04:29





Computers and Technology, 08.04.2020 04:29



History, 08.04.2020 04:29




Computers and Technology, 08.04.2020 04:29