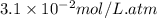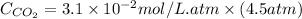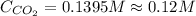Chemistry, 14.02.2020 02:27 cookie42087
Calculate the concentration of CO2 in water at 25 degrees Celsius when the pressure of CO2 over the solution is 4.5 atm. At 25 degrees Celsius, the Henry's law constant for CO2 in water is 3.1 * 10^-2 mol/L*atm.
? M

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
Calculate the concentration of CO2 in water at 25 degrees Celsius when the pressure of CO2 over the...
Questions



History, 09.03.2021 05:20








Arts, 09.03.2021 05:20



Physics, 09.03.2021 05:20




Mathematics, 09.03.2021 05:20

Advanced Placement (AP), 09.03.2021 05:20

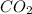 is, 0.12 M
is, 0.12 M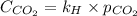
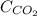 = concentration of
= concentration of 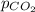 = partial pressure of
= partial pressure of 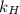 = Henry's law constant =
= Henry's law constant =