Chemistry, 14.02.2020 02:48 seiglersteven99
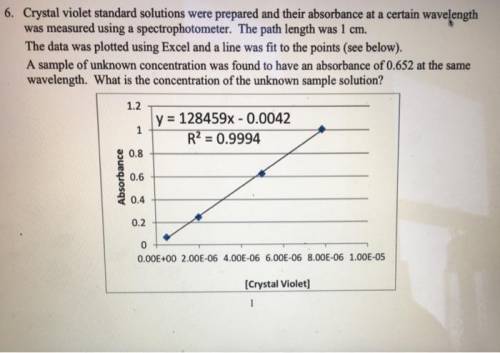
Crystal violet standard solutions were prepared and their absorbance at a certain wavelength was measured using a spectrophotometer. The path length was 1 cm. The data was plotted using Excel and a line was fit to the points (see below). A sample of unknown concentration was found to have an absorbance of 0.652 at the same wavelength. (a) What is the molar absorptivity of this compound at the certain wavelength? (b) What is the concentration of the unknown sample solution?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2
You know the right answer?
Crystal violet standard solutions were prepared and their absorbance at a certain wavelength was mea...
Questions


Mathematics, 21.01.2020 14:31

Chemistry, 21.01.2020 14:31



History, 21.01.2020 14:31



Mathematics, 21.01.2020 14:31

History, 21.01.2020 14:31

Mathematics, 21.01.2020 14:31

Chemistry, 21.01.2020 14:31



Mathematics, 21.01.2020 14:31


Mathematics, 21.01.2020 14:31


History, 21.01.2020 14:31