
Chemistry, 14.02.2020 02:44 SKSKSKSKGKUFHjk
Write the equilibrium-constant expression for the reactionA(s)+3B(l)↽−−⇀2C(aq)+D(aq)A (s)+3B(l)↽−−⇀2C(aq)+D(aq)in terms of [A], [B], [C], and [D] as needed. Note that KcKc, which is sometimes symbolized as KeqKeq, denotes that the equilibrium constant is expressed using molar concentrations. For this question, KcKc means the same thing as KeqKeq. Kc=.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2
You know the right answer?
Write the equilibrium-constant expression for the reactionA(s)+3B(l)↽−−⇀2C(aq)+D(aq)A (s)+3B(l)↽−−⇀2...
Questions


English, 19.04.2021 18:20

Physics, 19.04.2021 18:20

Health, 19.04.2021 18:20

Mathematics, 19.04.2021 18:20

Mathematics, 19.04.2021 18:20



English, 19.04.2021 18:20



Mathematics, 19.04.2021 18:20

Health, 19.04.2021 18:20

Mathematics, 19.04.2021 18:20

Computers and Technology, 19.04.2021 18:20



Mathematics, 19.04.2021 18:20

Engineering, 19.04.2021 18:20

Mathematics, 19.04.2021 18:20

![K_{c} = [\text{C}]^{2}[\text{[D]}](/tpl/images/0511/0111/07ffc.png)
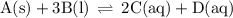
![K_{c} = \dfrac{[\text{Products}]}{[\text{Reactants}]}](/tpl/images/0511/0111/68d06.png)
![K_{c} = [\textbf{C}]^{\mathbf{2}}\textbf{[D]}](/tpl/images/0511/0111/3e70c.png)



