Chemistry, 14.02.2020 03:01 cordovatierra16
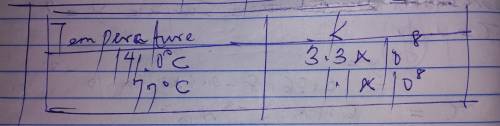
The rate constant for a certain reaction is measured at two different temperatures: temperature Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy for this reaction. Round your answer to significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
The rate constant for a certain reaction is measured at two different temperatures: temperature Assu...
Questions

Biology, 09.02.2022 14:00




Computers and Technology, 09.02.2022 14:00



English, 09.02.2022 14:00

SAT, 09.02.2022 14:00







Mathematics, 09.02.2022 14:00