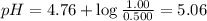Chemistry, 14.02.2020 03:28 andydiaz1227
What is the pH of a solution that is 0.500 M in acetic acid and 1.00 M in CH3COONa? Ka = 1.75*10-5 Group of answer choices 4.47 5.06 4.77 0.3 Flag this Question

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

You know the right answer?
What is the pH of a solution that is 0.500 M in acetic acid and 1.00 M in CH3COONa? Ka = 1.75*10-5 G...
Questions

History, 07.10.2019 20:30

Social Studies, 07.10.2019 20:30

Biology, 07.10.2019 20:30

Computers and Technology, 07.10.2019 20:30

Mathematics, 07.10.2019 20:30

Physics, 07.10.2019 20:30




Mathematics, 07.10.2019 20:30

Mathematics, 07.10.2019 20:30

Mathematics, 07.10.2019 20:30






Biology, 07.10.2019 20:30