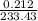Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

You know the right answer?
A solution contains an unknown mass of dissolved barium ions. When sodium sulfate is added to the so...
Questions

Mathematics, 18.03.2021 01:10



English, 18.03.2021 01:10


Social Studies, 18.03.2021 01:10

History, 18.03.2021 01:10




Mathematics, 18.03.2021 01:10




Arts, 18.03.2021 01:10


Social Studies, 18.03.2021 01:10

English, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10

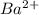 and
and 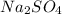 are added then white precipitate forms. And, reaction equation for this is as follows.
are added then white precipitate forms. And, reaction equation for this is as follows.
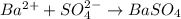
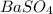 is 233.43.
is 233.43.