
You double the partial pressure of a gas over a liquid at constant temperature. Which of these statements is then true? a. The Henry’s Law constant is decreased by half. b. There are half as many gas molecules in the liquid. c. There is no change in the number of gas molecules in the liquid. d. The Henry’s Law constant is doubled. e. There are twice as many gas molecules in the liquid.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

You know the right answer?
You double the partial pressure of a gas over a liquid at constant temperature. Which of these state...
Questions

Mathematics, 21.08.2019 14:20

Mathematics, 21.08.2019 14:20


History, 21.08.2019 14:20




English, 21.08.2019 14:20

Mathematics, 21.08.2019 14:20

English, 21.08.2019 14:20

English, 21.08.2019 14:20



Spanish, 21.08.2019 14:20

Biology, 21.08.2019 14:20



Mathematics, 21.08.2019 14:20


Biology, 21.08.2019 14:20

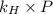
 = Henry's constant
= Henry's constant

