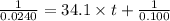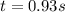Chemistry, 14.02.2020 06:11 cchavcchav2944
At a certain temperature the rate of this reaction is second order in with a rate constant of Suppose a vessel contains at a concentration of . Calculate how long it takes for the concentration of to decrease to . You may assume no other reaction is important. Round your answer to significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
At a certain temperature the rate of this reaction is second order in with a rate constant of Suppos...
Questions


Mathematics, 21.03.2021 02:10




Spanish, 21.03.2021 02:10

Spanish, 21.03.2021 02:10

Chemistry, 21.03.2021 02:10

Chemistry, 21.03.2021 02:10


Social Studies, 21.03.2021 02:10




Computers and Technology, 21.03.2021 02:10

English, 21.03.2021 02:10


Social Studies, 21.03.2021 02:10


Mathematics, 21.03.2021 02:10

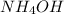 with a rate constant of
with a rate constant of 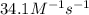 .
. 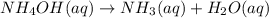
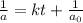
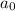 = initial concentration = 0.100 M
= initial concentration = 0.100 M