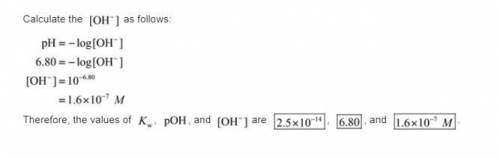. Like any equilibrium constant, Kw, changes with temperature. (a) Given that autoionization is endothermic, how does Kw change with rising T? Explain with a reaction that includes heat as a reactant or product. (b) In many medical applications, the value of Kw at 37°C (body T) may be more appropriate than the value at 25°C, 1.0x10-14. The pH of pure at 37°C is 6.80. Calculate Kw, pOH and [OH-] at this T.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
. Like any equilibrium constant, Kw, changes with temperature. (a) Given that autoionization is endo...
Questions



History, 26.11.2020 04:50

World Languages, 26.11.2020 04:50

Mathematics, 26.11.2020 04:50

Mathematics, 26.11.2020 04:50



Mathematics, 26.11.2020 04:50




History, 26.11.2020 04:50

Mathematics, 26.11.2020 04:50




Social Studies, 26.11.2020 04:50

Geography, 26.11.2020 04:50

Physics, 26.11.2020 04:50

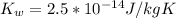
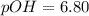
![[OH^{-}] =1.6*10^{-7} M](/tpl/images/0511/5212/a2f6d.png)