
Chemistry, 14.02.2020 17:26 danidavis2002
You have 50 ml of a complex mixture of weak acids that contains some HF (pKa = 3.18) and some HCN (pKa = 9.21). Which is larger, [F-]/[HF] or [CN-]/[HCN]?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

You know the right answer?
You have 50 ml of a complex mixture of weak acids that contains some HF (pKa = 3.18) and some HCN (p...
Questions



Biology, 25.05.2021 19:30

Mathematics, 25.05.2021 19:30



Mathematics, 25.05.2021 19:30




Mathematics, 25.05.2021 19:30

Mathematics, 25.05.2021 19:30

English, 25.05.2021 19:30




Chemistry, 25.05.2021 19:30

Computers and Technology, 25.05.2021 19:30


![\frac{[F^{-}]}{[HF]}](/tpl/images/0511/5531/fe465.png) is larger
is larger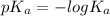 , where
, where 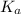 is the acid dissociation constant.
is the acid dissociation constant.![K_{a}=\frac{[H^{+}][A^{-}]}{[HA]}](/tpl/images/0511/5531/c814f.png) and
and ![\frac{[A^{-}]}{[HA]}=\frac{K_{a}}{[H^{+}]}](/tpl/images/0511/5531/b8841.png)
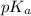
![[H^{+}]](/tpl/images/0511/5531/85507.png) will be constant.
will be constant.![(\frac{F^{-}}{[HF]}=\frac{K_{a}(HF)}{[H^{+}]})(\frac{CN^{-}}{[HCN]}=\frac{K_{a}(HCN)}{[H^{+}]})](/tpl/images/0511/5531/19b23.png)


