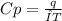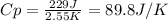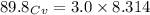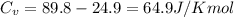Chemistry, 14.02.2020 17:25 postorivofarms
When 229 J of energy is supplied as heat to 3.0 mol of nitrogen N2(g) at constant pressure, the temperature of the sample increases by 2.55 K. Assuming that under these conditions nitrogen behaves as an ideal gas, what is the value of the molar heat capacity at constant volume of N2(g)?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 23.06.2019 02:00
What are fossils of organisms that existed over a wide area but only for a limited time period called?
Answers: 2
You know the right answer?
When 229 J of energy is supplied as heat to 3.0 mol of nitrogen N2(g) at constant pressure, the temp...
Questions




Mathematics, 23.04.2020 21:17

Mathematics, 23.04.2020 21:18



Chemistry, 23.04.2020 21:18


Social Studies, 23.04.2020 21:18

Mathematics, 23.04.2020 21:18


English, 23.04.2020 21:18



Social Studies, 23.04.2020 21:18



Mathematics, 23.04.2020 21:18

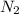 is 64.9 J/Kmol
is 64.9 J/Kmol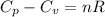
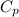 = heat capacity at constant pressure
= heat capacity at constant pressure  = heat capacity at constant volume
= heat capacity at constant volume