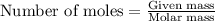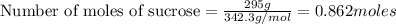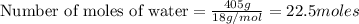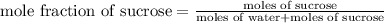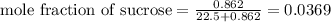Chemistry, 14.02.2020 18:55 AbhiramAkella
What is the mole fraction sucrose when 295 g sucrose C 12H 22O 11 (molar mass 342.3 g/mol) is dissolved in 405 g water?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

You know the right answer?
What is the mole fraction sucrose when 295 g sucrose C 12H 22O 11 (molar mass 342.3 g/mol) is dissol...
Questions

History, 19.01.2020 02:31


Mathematics, 19.01.2020 02:31

Social Studies, 19.01.2020 02:31



Chemistry, 19.01.2020 02:31

History, 19.01.2020 02:31



Mathematics, 19.01.2020 02:31


Biology, 19.01.2020 03:31



Mathematics, 19.01.2020 03:31

English, 19.01.2020 03:31

English, 19.01.2020 03:31